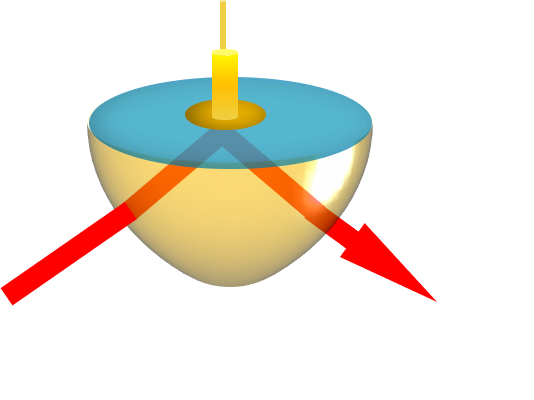
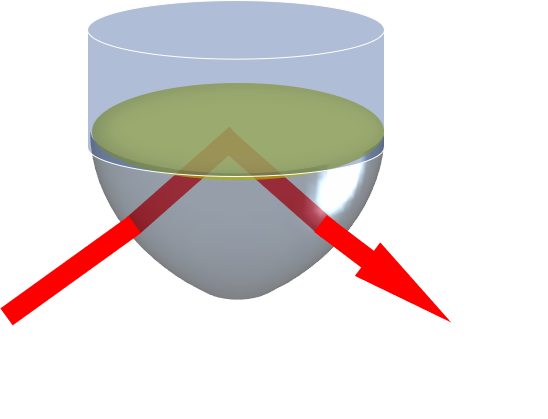
ATR-SEIRAS exploits the near-field interaction between incident infrared radiation and a thin metal film electrode, often a layer of gold. Working in the Kretschmann configuration, the surface of the metal layer can be probed under a variety of electrochemical conditions.
But how are metal layers for ATR-SEIRAS prepared? This post will describe the two main fabrication methods, physical vapour deposition and chemical (electroless) deposition. It will also describe a newly-developed method for preparing mechanically stable hybrid gold films using electrodeposition.
A general observation in ATR-SEIRAS is that metal films have very poor mechanical stability. Because of the poor adhesion of gold on silicon oxide surfaces, film delamination is the dominant failure mode. Metal films prepared by vapour or electroless should be handled very carefully. Prolonged potential excursions into gas evolution (highly anodic or cathodic potentials) will often result in high film stresses which contribute to failure.
Physical Vapour Deposition
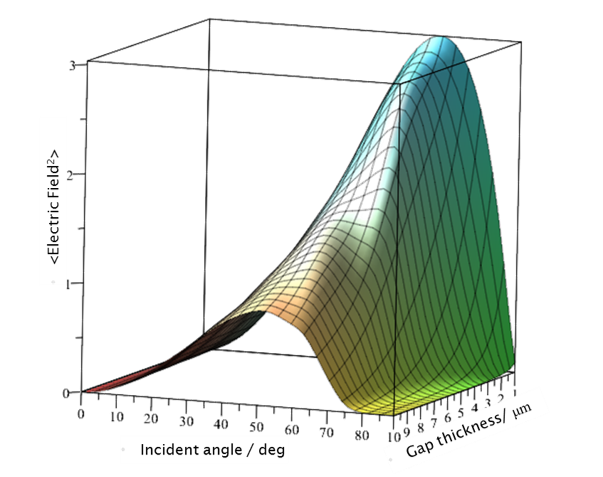
Physical Vapour Deposition (PVD) methods generally use magnetron sputtering or thermal evaporation systems. Metal thin films in the 10 to 20 nm thickness range are deposited at low rates (typically around 0.01 nm/s) on the basal plane of an internal reflection element. The low deposition rate and thickness provide a rough surface with an island structure which is known to contribute favourably to signal enhancement.
From a practical standpoint, PVD methods are relatively quick and usually highly repeatable with a well-maintained system. Because the films are prepared in vacuum, it often takes several hours to pump the substrates down to base pressure. Deposition usually takes under an hour. Most PVD chambers can accommodate multiple substrates, allowing several metal layers to be prepared in parallel. This is particularly beneficial when using our microgrooved wafers, available for purchase in our web store.
Although metal deposition with PVD methods is relatively quick and easy, the layers can be less mechanically stable than those prepared with chemical (electroless) methods. In our experience, enhancement factors are often slightly lower as well.
Chemical (Electroless) Deposition
Electroless deposition using gold on a silicon substrate was first described by Osawa in 2002, and involves a series of mechanical and chemical steps. Compared to PVD, it is highly challenging and potentially hazardous, but results in robust films with good enhancement factors. The basal plane of the internal reflection element is first mechanically polished with diamond suspensions (decreasing to sub-micron particle sizes) then treated with HF to remove the native oxide layer and hydrogen terminate the surface. Then, a plating solution containing a gold precursor (often gold tetrachloroaurate) is introduced and allowed to react for one to two minutes before the reaction is quenched by rinsing with water.
We find that in the hands of a skilled practitioner, this method generates relatively robust films with strong enhancement. However, even an experienced user will only have about a 50% success rate. Furthermore, the highly corrosive solutions involved require extreme care in use and should be treated with appropriate caution. Deposition in this manner can only be performed one crystal at a time. In total, including preparation and cleanup, electroless deposition usually takes around two hours to complete.
Another option that has been reported in the literature is electroless deposition on ZnSe internal reflection elements. PIKE Technologies offers ZnSe face-angled crystals which are compatible with our JxF-series cells should you wish to try this.
Because of the difficulty and chemical hazards involved, new users should strongly consider avoiding electroless deposition if they have the resources to do so.
Hybrid Metal / Conductive Metal Oxide Films
JackfishSEC workers have recently demonstrated the preparation and use of hybrid films for ATR-SEIRAS. In Andvaag et al., a 50 nm film of indium zinc oxide (IZO) was deposited by magnetron sputtering and then gold nanoparticles were electrodeposited in the presence of a shape-directing ligand (4-methoxypyridine). Such films have remarkably high enhancement and are robust enough to withstand repeated potential cycles into hydrogen evolution. The paper linked above illustrates the stability of the hybrid film by reductively desorbing a thiol monolayer three times from the same film with no meaningful change in the spectrum quality. This is essentially impossible on a conventional gold film.
Hybrid films, which can also use indium tin oxide (ITO), require access to a PVD chamber to prepare the conductive metal oxide (CMO) layer. Electrodeposition can be completed in situ in a spectroelectrochemical cell so that the enhancement can be monitored during the process. Although multiple CMO layers can be deposited in parallel, electrodeposition takes place in series, although it takes only around an hour to complete.
“Activating” A Metal Layer
Most of the literature describes a potential cycling routine to “activate” the metal layer. The exact processes involved are still unclear, but the empirical result is that the signal enhancement increases during the process. This will be covered in detail in a future post.