With the release of the J2 cell, you may be wondering which Jackfish cell is the most suitable for your research. The table below contrasts the features of the two cells to help you decide which cell might be a better fit.
| J1 | J2 | |
|---|---|---|
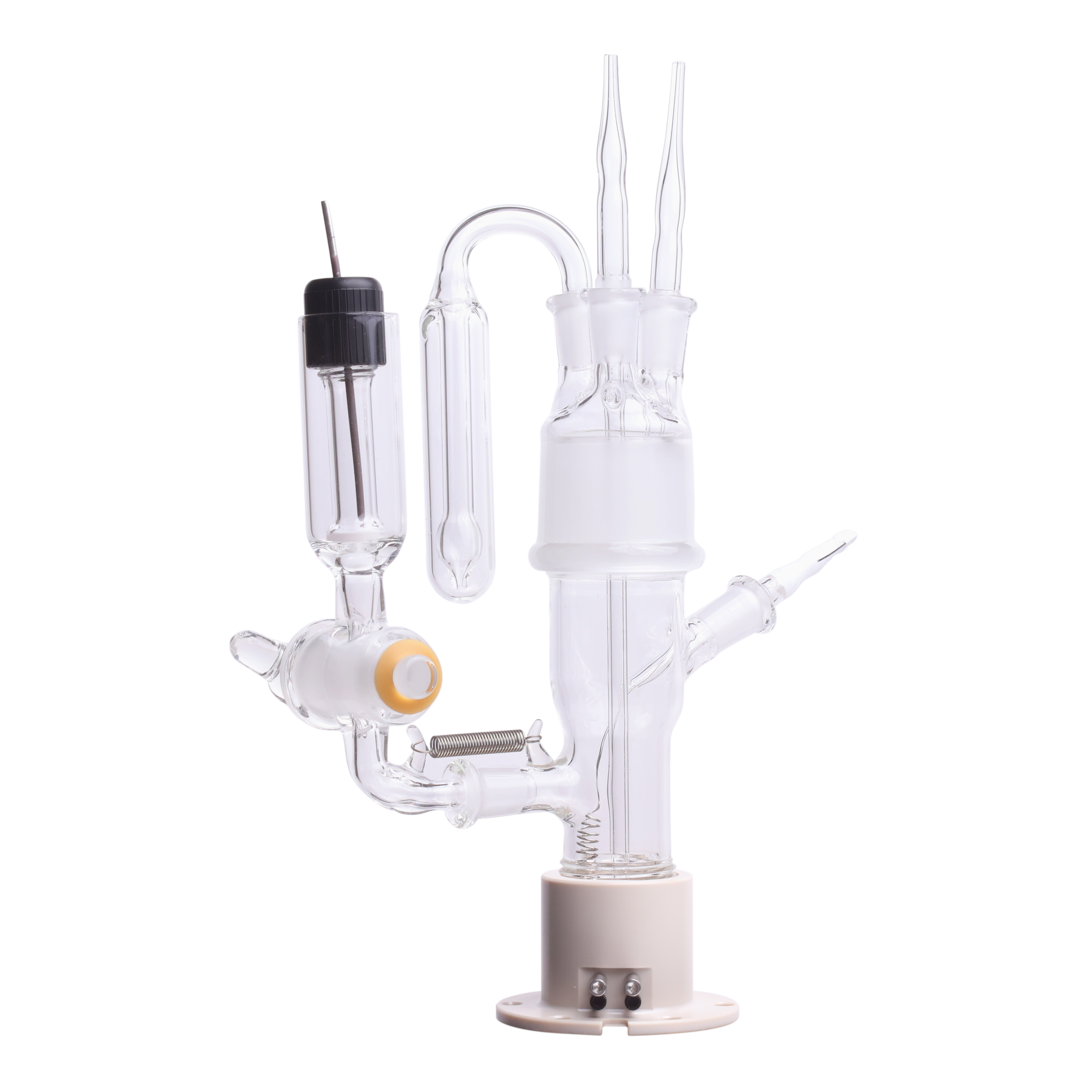 |  | |
| Material Purity | All wetted components made of glass, FKM/FFKM, and PTFE/PEEK. All components can be rigorously cleaned according to standard electrochemistry procedures. | Under normal use, the wetted materials are glass, FKM/FFKM, and PTFE/PEEK. The PTFE-coated rubber septum is exposed to solvent vapours. Celcon compression caps cannot be acid washed. |
| Electrolyte options | Ground glass joints designed for use with aqueous electrolyte. | Completely sealed cell design is compatible with volatile solvents. |
| Counter electrode | Jackfish offers a choice of Pt or Au CE. Extra 7/14 ground glass joints are supplied for users to utilize a metal wire of their choice. | Pt CE available as an add-on. Standard 6 mm diameter compression-style fitting accommodates a variety of third-party electrodes. |
| Reference electrode | Reference arm is separated with a stopcock which acts as a salt bridge, preventing migration of RE filling solution into the cell body. Jackfish offers Ag/AgCl RE, or the user can supply their own to fit in the 18 mm ID reference arm. | Electrodes are inserted directly into the cell in close proximity to the working electrode, reducing the impedance. A standard 6 mm diameter compression-style threaded port accommodates a variety of aqueous and non-aqueous commercial electrodes. |
| Air-sensitive chemistry | Only possible by greasing the ground glass joints. Wetted ground glass joints slowly evaporate water to the environment and are unsuitable for use in a glovebox. | Completely sealed design can be used with Schlenk technique, or in a glovebox. |
| Minimum cell volume | 20 mL (including reference arm) | 10 mL |
You might also want to check out our product release video for the J2 which walks through the differences between the cells in a bit more detail.
If you can’t decide which cell is best for you, or if you want to take advantage of the features of both cells, we do offer a combo discount available if both cells are purchased at the same time.